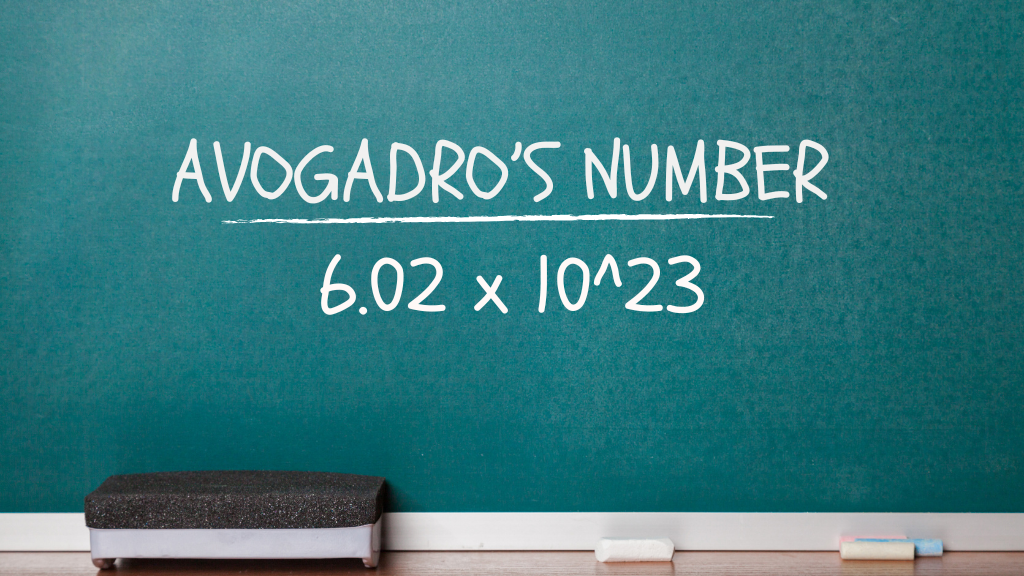
National Mole Day is an unofficial holiday celebrated by chemists on October 23rd from 6:02 a.m. to 6:02 p.m., based on Avogadro’s number (2$6.02 \times 10^{23}$), the number of particles in one mole of a substance.
Here are some excellent ways to integrate it into your classroom:
I. Introducing the Mole Concept4
The main goal is to make the mole understandable, as it’s just a unit of counting, like a dozen.5
- The “Dozen” Analogy: Start with concepts students already know.6 Ask: “Which is heavier, a dozen elephants or a dozen ping-pong balls?” A mole is simply a specific, very large counting number (7$6.02 \times 10^{23}$), just as a dozen is the number 12.8 Use this to illustrate that while a mole of any substance has the same number of particles, it will have a different mass (just like a dozen eggs and a dozen cars).9
- Mass-to-Number Relationship: Introduce the idea of counting by weighing.
- Activity Idea: Give students a large container of small, uniform items (like paperclips, beans, or beads). Have them:
- Determine the average mass of one item.
- Find the total mass of the container’s contents.
- Calculate the total number of items without counting them all.
- Relate this to how chemists use the molar mass (the mass in grams of one mole of a substance) from the periodic table to “count” the tiny atoms and molecules.
- Activity Idea: Give students a large container of small, uniform items (like paperclips, beans, or beads). Have them:
- The Scale of Avogadro’s Number: Use creative analogies to show just how huge $6.02 \times 10^{23}$ is. For example, if you had a mole of dollar bills, and you spent a billion dollars a second, it would still take you millions of years to spend it all!
II. National Mole Day Celebration Activities
Lean into the fun, pun-filled celebration to build excitement for chemistry.
- Creative Mole Projects: Assign creative, cross-curricular projects to be completed by Mole Day (October 23rd).10
- “Pet Moles”: Have students design, draw, or craft an adorable mole (the animal) with a chemistry-related pun or an Avogadro’s number fact.11 Example Puns: “Hol-E Mole-Y,” “Guaca-Mole,” “Mole-y Grail,” “Molympics.”12
- Mole Songs/Raps: Challenge students to write song parodies or raps that incorporate the mole concept, Avogadro’s number, and dimensional analysis.
- Mole Posters/Infographics: Students research Amedeo Avogadro and the history of the mole, creating educational posters to decorate the classroom or hallway.13
- Edible Chemistry: Incorporate food (if school policy allows).14
- Guaca-MOLE: Make or bring in guacamole.
- Mole-asses Cookies: Serve cookies or other mole-themed snacks.15
- Calculations and Competitions (Molympics):
- “How Big is a Mole?” Lab: Have students calculate the number of moles/molecules in common household substances like salt, sugar, water, or chalk by weighing small samples.16
- “Guess the Moles” Contest: Fill a beaker or container with an unknown amount of a compound (like copper sulfate).17 Have students predict the number of moles or atoms inside for a prize.
- Molympics: Organize friendly, mole-themed games or relays.18 Examples include water-bottle flipping relays (related to volume of a mole of gas) or a scavenger hunt solving mole conversion problems.19
These activities use the unique holiday to make a foundational, often challenging, chemistry concept like the mole engaging, memorable, and fun.
There are several ways to celebrate this scientific day. Do you know how to use the mole in an equation?
- Learn more about molecular science and Avogadro’s number.
- Explore the international system of measurement using moles.
- Test your knowledge of chemistry. Celebrate with other chemists and chemistry students.
- Conduct a mole experiment. While conducting it, see how many puns you can tell.
- In your classroom, do a video with your students demonstrating what a mole is.
- Create a rap about the mole. Be sure to include a little history of Amedeo Avogadro.
- While reading up on Italian scientist Amedeo Avogadro, explore other chemists of his era.
- Wear a t-shirt with 6.02×10^23 on it.